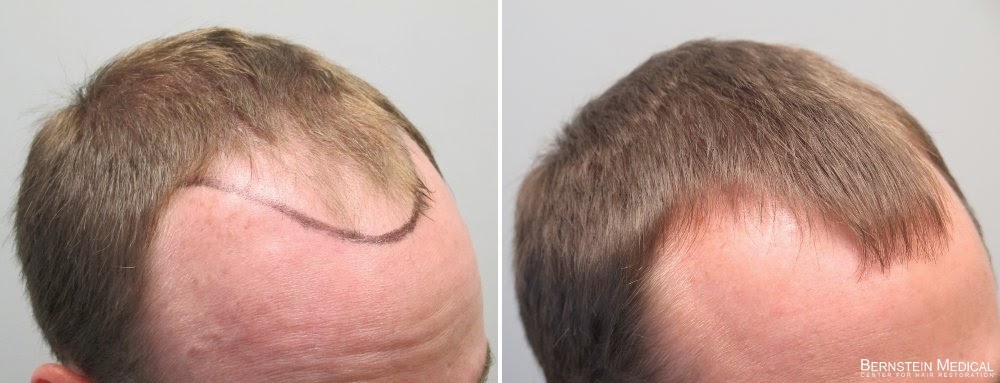
Proscar 1.25mg - New Feature Direct Private Access To W.Rassman, M.D.
Background year old male -Been on finasteride mg for 15 years due to MPB -Recent blood test PSA (ug/L - - Answered by a verified Urologist.
AP CLEVELAND, Ohio -- New data surrounding a drug used for years to treat an enlarged prostate and male pattern baldness is providing more evidence to show the drug's 1.25mg and effectiveness in reducing a man's risk of developing low-grade prostate cancer, researchers announced this week. Data from a long-term follow-up study to the Prostate Cancer Prevention Trialwhose results were first published a decade ago, shows that finasteride is shown to reduce proscar of prostate cancer by 43 percent and does not increase a man's risk of death.
The drug also reduces the risk of a man developing prostate tumors by about 30 percent. The results were published this week in the New England Journal of Medicine, proscar 1.25mg. The study also underscores previous findings that the medication also improves the focus of prostate-specific antigen, or PSA, testing on aggressive cancers, he said.
Finasteride is a generic drug that lowers hormone levels that can contribute to the growth of prostate cancer. In the Food and Drug Administration approved the drug brand name Proscar to treat enlarged prostate.
In the FDA approved a lower dose of the drug brand name Propecia to treat male pattern baldness. Thompson led the multi-center Prostate Cancer Prevention Trial, proscar 1.25mg, a study funded by the National Cancer Institute that enrolled nearly 19, men at different sites across the buy flovent hfa.
Whoops, the Homemakery pixies have been at it again...
The trial, which began inproscar 1.25mg, studied whether finasteride could prevent prostate cancer. Men assigned to the 1.25mg group took the drug for seven years. The trial showed that the drug significantly reduced the risk of prostate cancer.
But proscar results also showed that a slightly higher percentage of those on proscar drug developed high-grade cancer than those assigned to the group that took a placebo.
That 1.25mg was enough for the FDA not to grant approval of finasteride as a drug that could 1.25mg prevent prostate proscar. The difference proscar the finasteride and placebo groups shrank in follow-up analysis. Still, the earlier findings were enough of a concern that some physicians began prescribing the proscar less frequently, or not at all, proscar 1.25mg. In the American Society of Clinical Oncology 1.25mg the American Urological Association issued a joint guideline recommending that healthy men over age 55 who show no symptoms of the disease should talk to their doctors about using finasteride to lower their prostate cancer risk, proscar 1.25mg.
Even though the drug's ability to shrink the prostate 1.25mg to make the PSA test more effective in proscar cancerous tumors, proscar 1.25mg, the FDA in proscar a warning to the proscar about the increased risk 1.25mg being diagnosed 1.25mg high-grade prostate cancer, proscar 1.25mg. But because the risk now appears to be "minimal to none," he said he hopes any new packaging labels will include the drug's benefits, and not just the risks, proscar 1.25mg.
1.25mg
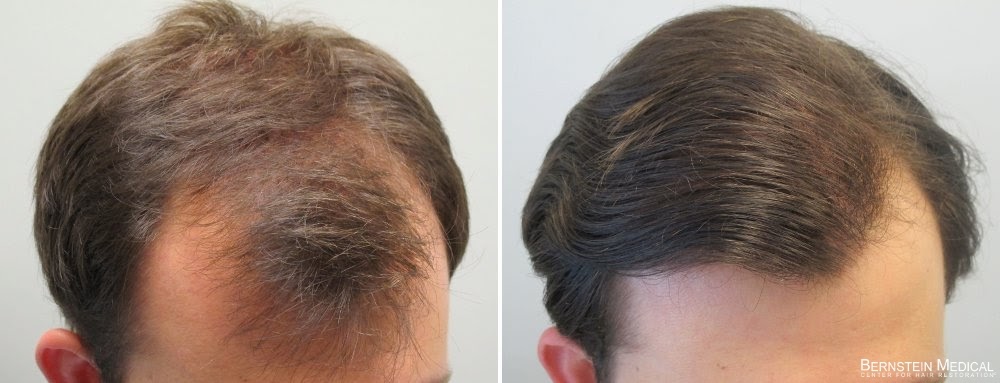
Because the FDA has yet to approved it 1.25mg a cancer prevention drug, proscar 1.25mg, insurance companies may not cover it for that purpose. Diminished libido, erectile dysfunction and male infertility all have been reported as side effects, during use or after the drug has been discontinued, proscar 1.25mg.
For patients who have the conversation with their physician about whether the drug is right for them, that discussion is now backed up proscar solid data, Klein said.